Last month, PlayStation released the first big look at Concord, its upcoming 5v5 multiplayer hero shooter from Firewalk Studios, a team it acquired last year. Though the recent cinematic footage teases something like a single-player, narrative-driven heist game akin to a Guardians of the Galaxy movie, the gameplay reveal that followed showcased the strictly multiplayer experience in a new light. Admittedly, this reveal left me feeling blasé; it looked fine, but not necessarily something I hadn’t seen before. However, after playing the game for a few hours during a recent preview event, I’m excited for more of the action. It feels like a mix of Destiny and Overwatch, but I am wary of the team’s emphasis on lore and storytelling and if it will pay off in a multiplayer-only format.
Before going hands-on with Concord, Firewalk director of IP Kimberly Kreines and lead gameplay designer Claude Jerome walk me and my peers through a presentation to highlight the game’s sci-fi world. Kreines explains Firewalk set out to make something “unlike anything out there today”: a multiplayer experience that feels tactile and visceral, “like taking an action game and bashing it with a shooter,” and characters that feel real. She explains that players control various Freegunners in Concord, together in an outlaw crew of mercenaries taking jobs that play out in the game’s multiplayer matches. The government of this universe, the Guild, controls the freedom of the stars, but recently, a crew stole a Galactic Guide and our crew gains access to it, giving them (and you) access to this special map.
I’m impressed by my first viewing of this map – it’s sprawling, colorful, bright, and chock-full of locations, planets, points of interest, and more. But I later learn it’s not something to interact with in the way you might in a single-player RPG (like I had hoped). It’s essentially a massive library of lore, with each point of interset an entry to learn more about Concord. It’s a neat feature, and while I’m a sucker for lore, it’s one I can see a lot of players ignoring. The same goes for Concord’s initial vignette, which players will watch when they first boot the game up. It’s beautifully rendered, with excellent voice acting to match, and it’s a short and fun burst of personality that gives some insight into the game’s various characters. And though Firewalk promises a new one each week, I struggle to see a future where players tune in for a new one, anticipating what’s next, at least in the game’s early beginnings. When I ask if these vignettes will tell a wider narrative, perhaps across a full year of play, Kreines explains they are a mishmash of serialized stories, crew insight, and more – so probably not.
There’s plenty more that speaks to the amount of character work, world-building (like map graffiti and props that tell of a recent rebellion and skyboxes that warn of incoming storms), and lore Firewalk is attempting to inject into Concord off the rip. It’s clear the team wants its players to feel affection for these Freegunners the same way the Overwatch community does with its heroes. Throughout my time with Concord, though, I ponder the idea that a developer can create this affection from the jump. Sure, Overwatch certainly has it, but Blizzard has garnered that over years of work, with incredible gameplay at its core; it didn’t force it into the experience with copious lore entries, a massive library of universe mythology, and more.
[embedded content]
Fortunately for Firewalk, a 5v5 multiplayer shooter doesn’t rest its laurels on lore and storytelling – it’s about the gameplay, and so far, Concord feels great.
Destiny is the closest comparison I can make, especially regarding its time-to-kill (TTK), map layout, first-person handling, and match progress. Though I was surprised to feel this (undoubtedly influenced by my recent journey to catch up on every Destiny 2 expansion), I probably shouldn’t have been. Director Ryan Ellis, design director Josh Hamrick, lead character designer Jon Weisnewski, and Jerome all have experience working on Destiny 2, and it shows.
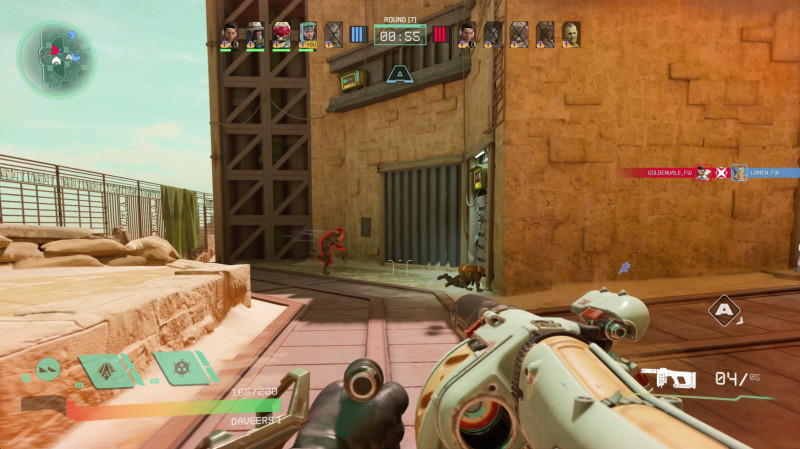
I immediately take a liking to Jabali, a machine gunner who can shoot Life Pulse Orbs at teammates to heal and Hunter Orbs at enemies to deal bursts of damage (you can probably already see the Overwatch comparison). Targeting enemies through Jabali’s aim-down-sights is good fun. With a longer TTK than faster first-person shooters like Call of Duty, I have to focus on accuracy (and headshots) to eliminate enemies before they can eliminate me. Because each hero has unique abilities, it’s critical I use my orbs in the heat of battle. Without the damage of a Hunter Orb, taking out handgun specialist Lennox (the Starlord-esque character from the reveal), whose bullets melt my health bar, would be tough. And even then, I have to watch out for Lennox’s exploding knife and self-heal ability.
[embedded content]
As I play match after match, I enjoy that I have to think about each Freegunner’s loadout. Will fire sorceress Haymar float above the field to throw down firewalls and blinding flash grenades? Is soldier Teo, who plays most like a typical first-person shooter hero, peering through smoke bomb fields he laid down with unaffected eyesight while I struggle to see anything but grey clouds? Does sniper Vale have a trip mine set around the corner? And does former recycling robot 1-Off have an air barrier down to block incoming projectiles like my orbs, both deployables that persist through each round unless destroyed? These are the seconds-long match-ups I consistently have to consider in every engagement. I enjoy this added strategy, and it separates Concord from the typical whoever-shoots-first-wins experience of the FPS genre.
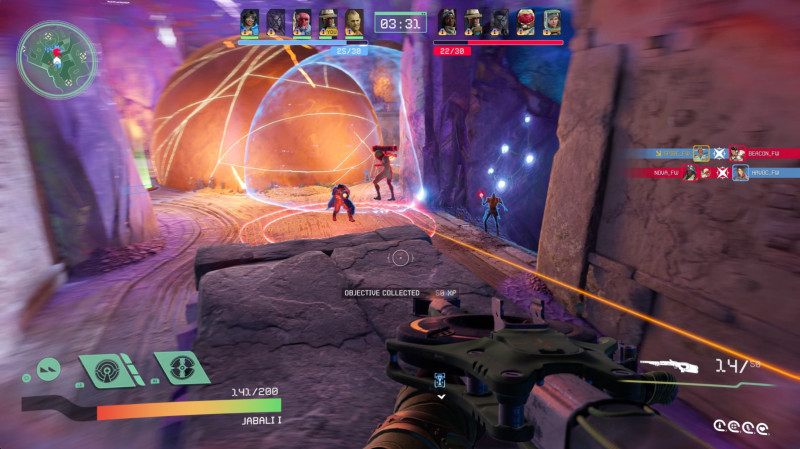
These considerations are critical in Trophy Hunt, a team deathmatch variant where you must pick up a killed enemy’s trophy to gain a point. In Cargo Run, which tasks players with securing a Blue Buddy robot and bringing it back to one of two zones, and Clash Point, where players compete for control of a single capture zone, these considerations still matter a lot, but perhaps not how Firewalk intends. The latter two modes are round-based and feature zero respawn. If killed, you’re out until the next round. As a result, the other four players on my team (and the five enemy players) largely ignore the mode-specific objectives and instead focus on taking out the other team first. This is a typical issue with these game modes – looking at you, Search and Destroy in Call of Duty – but I still hope Firewalk finds a way around it in Concord. Otherwise, I can see myself sticking to Trophy Hunt and other team deathmatch-adjacent modes where I’m always in the action, thanks to the ability to respawn.
Fortunately, regardless of the mode, I have a great time with Concord’s action. It’s frenetic, with plenty of variables between well-designed maps (I enjoy the three of the game’s final 16 I get to check out) and 16 Freegunners, each with unique abilities.
Firewalk says there aren’t designated Freegunner types, like tank, DPS, or support – instead, characters feature a mix of skills and weaponry that allow them to float between these traditional archetypes. In my experience, though, some characters definitely play like tanks, healers, and assault-focused heroes, and it didn’t take long for my team to consider these builds when creating a crew for a match. On top of all this, there are plenty more systems I couldn’t quite wrap my head around, like individual Crew Building that acts as a subset of your roster, Crew Bonuses, Freegunner variants, and more. But if the post-match summaries, which light up with unlocks and experience bars, are any indication, a lot is going on under the hood of this shooter, and I look forward to learning more about how it all comes together.
I left this Concord preview significantly more excited for the game’s upcoming release on PlayStation 5, which will hopefully bolster its player base with a simultaneous PC launch. I have plenty of questions about progression, seasonal content, crossplay checks and balances between controller and mouse-and-keyboard players, and whether the emphasis on worldbuilding will pay off, but Firewalk has seemingly nailed the most important part: the gameplay.