Among high-level sales, pharmaceuticals rank among the hardest products to sell, especially in today’s fast-paced market, where new and specialized drugs are approved every week. With this plethora of new drugs coming to the market, busy doctors have a hard time keeping up with new developments,…
The Vulnerabilities and Security Threats Facing Large Language Models
Large language models (LLMs) like GPT-4, DALL-E have captivated the public imagination and demonstrated immense potential across a variety of applications. However, for all their capabilities, these powerful AI systems also come with significant vulnerabilities that could be exploited by malicious actors. In this post, we…
Run, tune, and scale generative models that power AI applications
We’re going to talk about what OctoAI is and how we help our customers be successful, and then we’re going to take a look under the hood to see how it all works….
AI recruitment’s bias: Missing top talent
Explore how AI-driven hiring tools are inadvertently sidelining top talent, raising concerns over biases and the need for ethical AI practices….
From prototype to product with generative AI and large models
Shivani Poddar, Engineering Lead at Google, revisits the challenges with GenAI prototypes today and shares three strategies to overcome them. …
Investigating and preserving Quechua

Soledad Chango, a native of Ecuador and a graduate student in MIT’s Indigenous Language Initiative, began preparations for her Quechua course with a clear idea about its purpose.
“Our language matters,” she says. “It’s worth studying and spreading.”
Quechua at MIT, a new two-week introductory class hosted by MIT Global Languages during the Institute’s Independent Activities Period in January, introduced students to the basics of Kichwa, a Quechua variant that is the most widely spoken language in the Americas. The class, which featured an interactive approach, focused on oral and written skills, emphasizing tasks based on familiar contexts. “I prepared conversations that reflect cultural values,” Chango emphasizes.
Chango, a scholar of language acquisition, credited her advisor, MIT Linguistics professor Norvin Richards, and postdoc Cora Lesure with helping shape the course. Global Languages section head Per Urlaub helped ready the course for the classroom. “They helped me refine my ideas about what to teach and how to teach it,” she says.
Cultural immersion, value, and language acquisition
Because language can often be better understood when connected with its cultural context, Chango introduced students to the history, culture, and geography of the Andes mountains where the language’s speakers live, work, and play. Cultural discussions and interactions with artifacts were designed to help students understand the value of the endangered language.
“Every day, we dedicated time to individually review our writing and grammar skills,” says Isabel Naty Sanchez Taipe, a computer science and education student at Wellesley College and a cross-registered student and student researcher at MIT. “We practiced the pronunciation of new vocabulary and sentences out loud, and engaged in diverse group activities and games where we spoke Quechua as much as possible.”
Chango sought to emphasize the importance of keeping Kichwa and Quechua alive. When endangered languages disappear, so do the communities and culture from which they rose.
“In 2014, I was investigating Indigenous language advancement, tracking changes and usage,” she says. “Research shows the youngest Indigenous people retain and value their native languages the least.”
Multilingualism as a tool for improvement
Multilingualism can prove valuable both academically and professionally.
“I would definitely recommend that people explore languages taught in this manner,” says Prahlad Balaji Iyengar, a PhD student in electrical engineering and computer science who took the course. “I think this was a great opportunity for me to learn a new mode of thought.”
As Chango continues to review and refine the course, she looks to technology to both help retain Quechua’s distinctive traits and reverse its trajectory toward extinction. She wants to ensure languages like Kichwa find interested audiences outside of their native cultures.
“Technology can help spread the word and increase interest in Indigenous languages like Quechua,” she says. “I want to expand its reach from oral tradition and transmission and develop it so it supports quantifiable and replicable language instruction.”
How early-stage cancer cells hide from the immune system
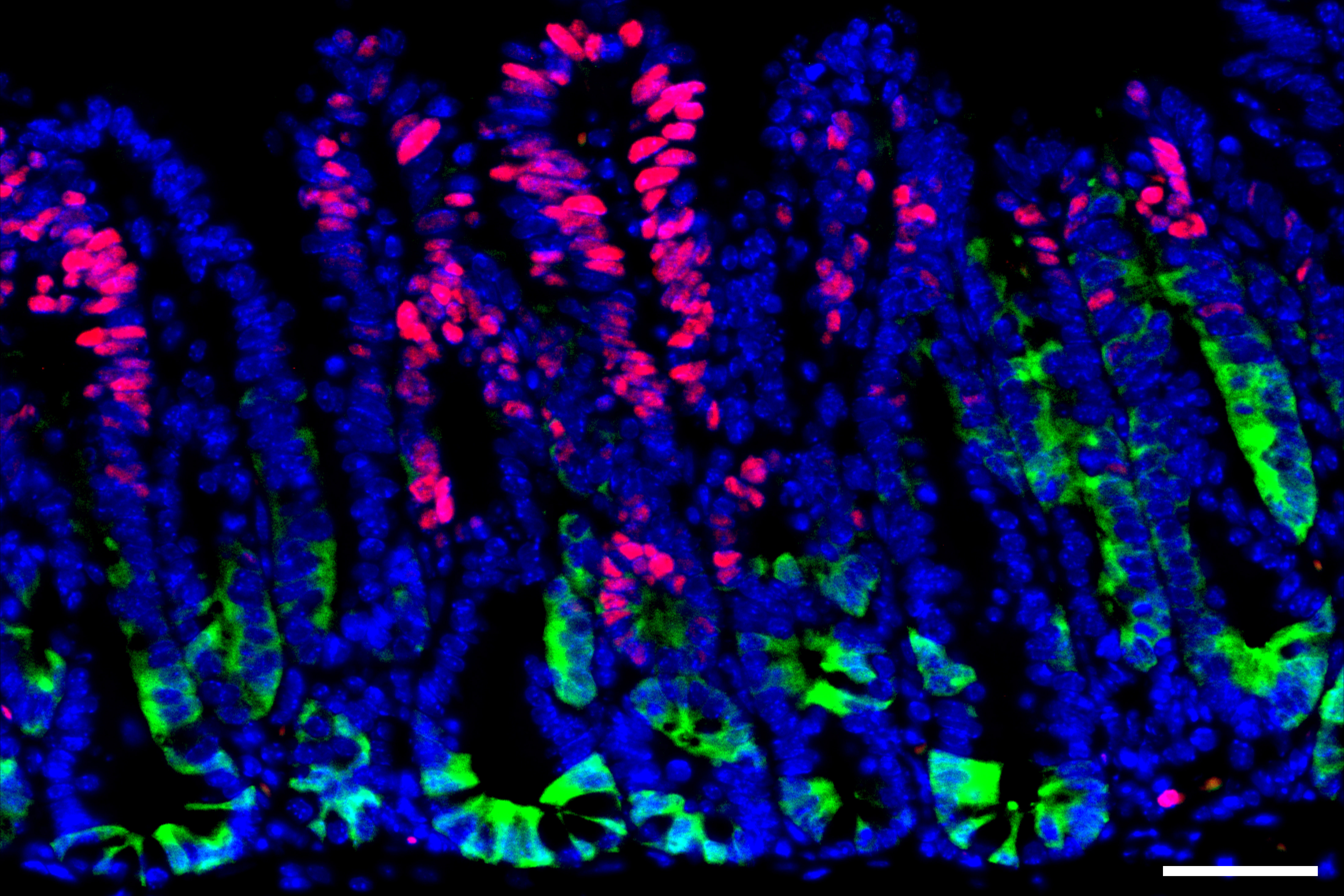
One of the immune system’s primary roles is to detect and kill cells that have acquired cancerous mutations. However, some early-stage cancer cells manage to evade this surveillance and develop into more advanced tumors.
A new study from MIT and Dana-Farber Cancer Institute has identified one strategy that helps these precancerous cells avoid immune detection. The researchers found that early in colon cancer development, cells that turn on a gene called SOX17 can become essentially invisible to the immune system.
If scientists could find a way to block SOX17 function or the pathway that it activates, this may offer a new way to treat early-stage cancers before they grow into larger tumors, the researchers say.
“Activation of the SOX17 program in the earliest innings of colorectal cancer formation is a critical step that shields precancerous cells from the immune system. If we can inhibit the SOX17 program, we might be better able to prevent colon cancer, particularly in patients that are prone to developing colon polyps,” says Omer Yilmaz, an MIT associate professor of biology, a member of MIT’s Koch Institute for Integrative Cancer Research, and one of the senior authors of the study.
Judith Agudo, a principal investigator at Dana-Farber Cancer Institute and an assistant professor at Harvard Medical School, is also a senior author of the study, which appears today in Nature. The paper’s lead author is MIT Research Scientist Norihiro Goto. Other collaborators include Tyler Jacks, a professor of biology and a member of MIT’s Koch Institute; Peter Westcott, a former Jacks lab postdoc who is now an assistant professor at Cold Spring Harbor Laboratory; and Saori Goto, an MIT postdoc in the Yilmaz lab.
Immune evasion
Colon cancer usually arises in long-lived cells called intestinal stem cells, whose job is to continually regenerate the lining of the intestines. Over their long lifetime, these cells can accumulate cancerous mutations that lead to the formation of polyps, a type of premalignant growth that can eventually become metastatic colon cancer.
To learn more about how these precancerous growths evade the immune system, the researchers used a technique they had previously developed for growing mini colon tumors in a lab dish and then implanting them into mice. In this case, the researchers engineered the tumors to express mutated versions of cancer-linked genes Kras, p53, and APC, which are often found in human colon cancers.
Once these tumors were implanted in mice, the researchers observed a dramatic increase in the tumors’ expression of SOX17. This gene encodes a transcription factor that is normally active only during embryonic development, when it helps to control development of the intestines and the formation of blood vessels.
The researchers’ experiments revealed that when SOX17 is turned on in cancer cells, it helps the cells to create an immunosuppressive environment. Among its effects, SOX17 prevents cells from synthesizing the receptor that normally detects interferon gamma, a molecule that is one of the immune system’s primary weapons against cancer cells.
Without those interferon gamma receptors, cancerous and precancerous cells can simply ignore messages from the immune system, which would normally direct them to undergo programmed cell death.
“One of SOX17’s main roles is to turn off the interferon gamma signaling pathway in colorectal cancer cells and in precancerous adenoma cells. By turning off interferon gamma receptor signaling in the tumor cells, the tumor cells become hidden from T cells and can grow in the presence of an immune system,” Yilmaz says.
Without interferon gamma signaling, cancer cells also minimize their production of molecules called MHC proteins, which are responsible for displaying cancerous antigens to the immune system. The cells’ insensitivity to interferon gamma also prevents them from producing immune molecules called chemokines, which normally recruit T cells that would help destroy the cancerous cells.
Targeting SOX17
When the researchers generated colon tumor organoids with SOX17 knocked out, and implanted those into mice, the immune system was able to attack those tumors much more effectively. This suggests that preventing cancer cells from turning off SOX17 could offer a way to treat colon cancer in its earliest stages.
“Just by turning off SOX17 in fairly complex tumors, we were able to essentially obliterate the ability of these tumor cells to persist,” Goto says.
As part of their study, the researchers also analyzed gene expression data from patients with colon cancer and found that SOX17 tended to be highly expressed in early-stage colon cancers but dropped off as the tumors became more invasive and metastatic.
“We think this makes a lot of sense because as colorectal cancers become more invasive and metastatic, there are other mechanisms that create an immunosuppressive environment,” Yilmaz says. “As the colon cancer becomes more aggressive and activates these other mechanisms, then there’s less importance for SOX17.”
Transcription factors such as SOX17 are considered difficult to target using drugs, in part because of their disorganized structure, so the researchers now plan to identify other proteins that SOX17 interacts with, in hopes that it might be easier to block some of those interactions.
The researchers also plan to investigate what triggers SOX17 to turn on in precancerous cells.
The research was funded by the MIT Stem Cell Initiative via Fondation MIT, the National Institutes of Health/National Cancer Institute, and a Koch Institute-Dana Farber Harvard Cancer Center Bridge Project grant.
Best Place to Buy Prebuilt Shopify Store in 2024 – Technology Org
Shopify is already a renowned e-commerce platform known as an excellent tool for developing an online store. For…
ChatGPT Acts More Altruistically and Cooperatively Than Humans – Technology Org
Modern artificial intelligence, such as ChatGPT, can mimic human behaviours, but the former has more positive outcomes, such…
Black Hole Fashions Stellar Beads on a String – Technology Org
An international team of astronomers has discovered one of the most powerful eruptions from a black hole ever…
